Orphan Drugs dominate the shortlist for the 2018 final – while medtech makes its first appearance
London, 5thJune, 2018. The pharmaceutical industry’s growing investment in rare diseases has been rewarded with a record number of orphan drugs making the shortlist for one of the most prestigious prizes in pharma R&D – a Prix Galien medal.
Seven orphan treatments have made the final of UK Prix Galien 2018, with one – Galafold (from Amicus) – becoming the first orphan drug to be shortlisted in both the Orphan Product and Innovative Product categories.
The 2018 ceremony will also award its first-ever prize for Medical Technology. However, in a move that suggests pharma still has some way to go in its application of Real World Evidence (RWE), the UK Prix Galien judges failed to shortlist any entrants in the RWE category that was first introduced in 2016.
UK Prix Galien 2018 will yet again take place at the House of Commons, London. The final includes representation from eight biopharmaceutical companies and one global medical technology company. The shortlist, which followed a rigorous review by UK Prix Galien’s independent judging panel, highlights the diverse and changing nature of UK life sciences as it grapples ongoing challenges and uncertainty around pricing, access and innovation in the lead up to Britain’s exit from the European Union. The shortlist is dominated by biotechs and specialist pharma companies.
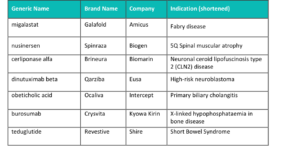
UK finalists
All products shortlisted in the Innovative Product and Orphan Product categories at UK Prix Galien 2018 were launched or granted a new indication in the UK between 1stJanuary 2016 and March 29th2018. The inaugural Medical Technology category was open to therapeutic products that received marketing approval in the UK in the five years up to March 29th2018, with additional provision made for CE-approved technologies that received approval in new or innovative indications within the previous five years.
Innovative Product Award
This year, the judges concluded that just two entrants were worthy of being shortlisted for the Innovative Product Award; Sanofi (Dupixent) and Amicus (Galafold).

The most recent winner of the Innovative Product Award, at the 2016 UK Prix Galien, was Novartis’ Entresto – the first new treatment for heart failure in 15 years.
Orphan Product Award
Seven companies have been shortlisted for the Orphan Product Award; Amicus (Galafold), Biogen(Spinraza), Biomarin(Brineura), Eusa (Qarziba), Intercept (Ocaliva), Kyowa Kirin (Crysvita) and Shire (Revestive).
The 2016 Orphan Drug Award was won by Holoclar, which in 2015 became the first stem cell therapy to receive European marketing approval. The therapy is used to treat moderate-to-severe forms of limbal stem cell deficiency (LSCD), a rare eye condition that can lead to blindness.
Medical Technology Award
Only one company was shortlisted for the inaugural Medical Technology Award; Medtronic (Solitaire Platinum). The Solitaire Platinum revascularization device is indicated for clot retrieval from occluded blood vessels in the brain due to ischemic stroke.
Commenting on the 2018 shortlist, Karen Westaway,Chief Executive of ValueBase – owners of Prix Galien’s UK franchise – said: “UK Prix Galien is always evolving and 2018 is no different. The introduction of a prize for Medical Technology is in recognition of the sector’s rich history in developing innovative devices, diagnostics and medical equipment. We hope that the category goes from strength to strength in future years – just like we’ve seen with orphan drugs. Orphan Products became a Prix Galien category in its own right ten years ago, following the award of a special prize in 2006. The past decade has seen a major shift in global pharma’s R&D model, with increased investment in the development of treatments for rare disease. In 2016, 41% of drugs approved by the FDA were for orphan conditions – and it’s a similar story in Europe. It’s wonderful to see companies continuing to commit to this important area of medicine – and equally pleasing to see this investment being reflected in the 2018 UK Prix Galien shortlist. It’s going to be a fiercely contested final. We look forward to welcoming UK pharma to the House of Commons in the autumn to find out who wins these prestigious prizes.”
